By Dr Jonas Nilsen and his team at Practio the infectious disease and travel vaccination specialists
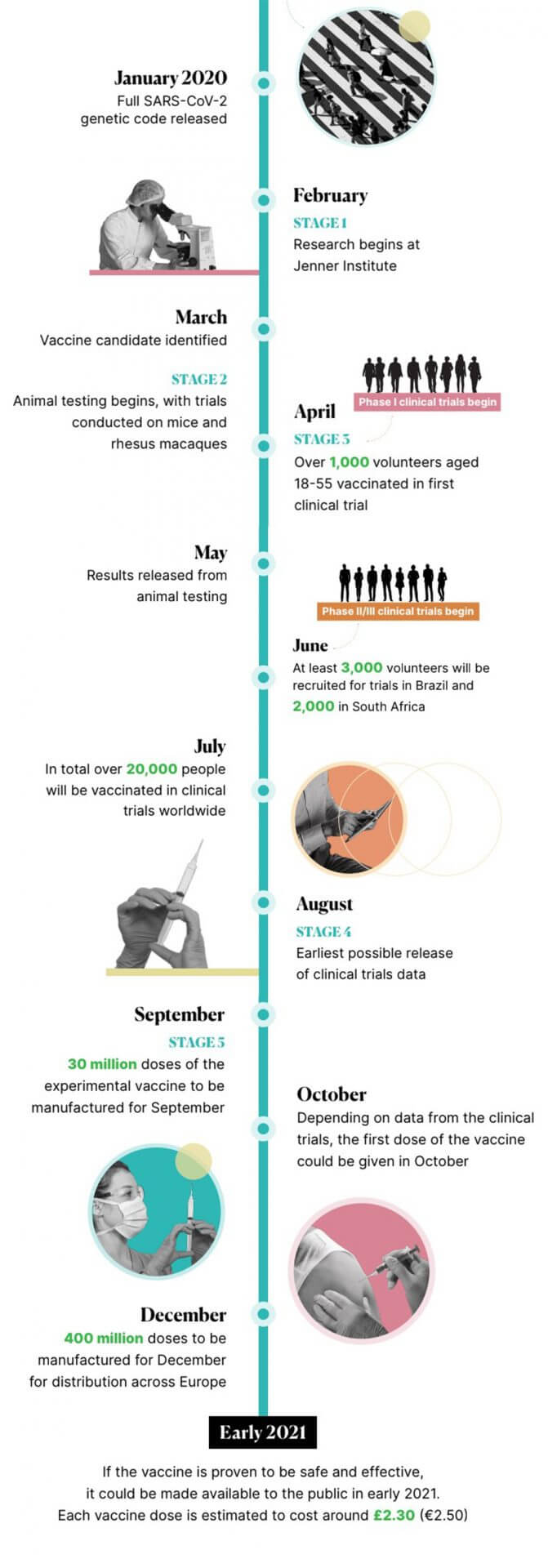
The University of Oxford’s Jenner Institute began work on a coronavirus vaccine in February. Development on the COVID-19 vaccine has progressed rapidly since, with human testing in clinical trials beginning in April. A lot of media attention has been paid to claims that the vaccine will be ready by autumn. In this article we’ll explain how the Oxford University COVID-19 vaccine works, current progress in its development, and how likely it is that it will be readily available by the end of this year.
The University of Oxford’s Jenner Institute began work on a coronavirus vaccine in February. Development on the COVID-19 vaccine has progressed rapidly since, with human testing in clinical trials beginning in April. A lot of media attention has been paid to claims that the vaccine will be ready by autumn. In this article we’ll explain how the Oxford University COVID-19 vaccine works, current progress in its development, and how likely it is that it will be readily available by the end of this year.
How does the Oxford COVID-19 vaccine work?
The Oxford COVID-19 vaccine has been developed using a method which was previously used to create a vaccine for another coronavirus, Middle East Respiratory Syndrome (MERS). The vaccine was shown to induce a strong immune response, meaning it would be likely to create immunity in those who received it. The MERS Oxford vaccine has not yet completed the clinical trials stage due to falling infection rates.
Like previous vaccines produced by the same institute, the ChAdOx1 nCoV-19 vaccine from the University of Oxford is made using an adenovirus. This is a milder virus with symptoms much like a common cold, which is manipulated so it cannot reproduce within the body and so it holds the genetic instructions to teach the body how to create coronavirus antibodies. The vaccine induces an immune response which creates the necessary antibodies that the body needs to fight coronavirus, creating immunity in the patients who receive it.
What is the current stage of development?
The Oxford vaccine is currently in the clinical trials stage of development, which is when groups of volunteers are given the vaccine in order to determine its safety and effectiveness.
Every vaccine must go through a number of different stages in development, usually starting with the exploratory stage, before moving on to the pre-clinical stage, which involves testing the vaccine either in a laboratory or on animals. Once the vaccine is determined to be safe to test on humans, and have a good chance of creating immunity, it can then be tested on humans.
In the case of the Oxford COVID-19 vaccine, after preliminary testing on animals, development went into to the human trials stage. Additional pre-clinical testing is being conducted in parallel with clinical trials.
Animal testing results
In addition to the vaccine being tested on groups of volunteers, tests have been made on rhesus macaques, which are considered to have a similar immune response to humans. The findings (which have not yet been peer-reviewed) showed that after being given one dose of the vaccine, this “significantly reduced viral load” in the monkeys, and they were protected from contracting coronavirus-related pneumonia in the lungs. However, they also still had just as much coronavirus genetic material in their noses as unvaccinated monkeys, meaning that all of the vaccinated and unvaccinated monkeys were infected with COVID-19.
Rhesus macaques have been used in pre-clinical testing to determine whether the Oxford COVID-19 vaccine is effective.
This could be for a number of reasons. It has been suggested by Dr Sarah Gilbert, one of the lead researchers developing the vaccine, that the high levels of coronavirus molecules present in the noses of the monkeys could be due to the fact that they received very high doses of the virus. It is also unclear as to whether the virus particles found in the noses of the monkeys were actively infectious, as they could also be “inactivated” byproducts of the body’s immune response.
Alternatively, it has been suggested that the vaccine can only produce immunity in the lungs, but not in the mucous membranes in the nose. According to some immunology experts, this has been the case with previous coronavirus vaccines.
It is impossible to tell for certain if the Oxford COVID-19 vaccine works until the full results from the clinical trials have been released and reviewed.
Human trial stage
Clinical trials are usually made up of three different phases. In phase I, small groups of volunteers receive the vaccine to ensure it is safe. In phase II, the effectiveness of the vaccine is determined with a larger group of volunteers. In phase III, an even larger group of volunteers receives the vaccine, which tests the effectiveness and safety of the vaccine on a diverse group of people of different ages and backgrounds. This ensures that the vaccine will work for everyone.
The Oxford COVID-19 vaccine was one of the first in the world to begin testing on groups of volunteers. Starting in April, combined phase I and II testing began when more than 1,000 volunteers received the experimental vaccine in the UK. Falling levels of coronavirus infection means that the phase II/III of clinical trials will be conducted in another country. As a result, the Brazilian Health Regulatory Agency has approved trials for the Oxford vaccine in Brazil.
Will the vaccine be ready by September?
Scientists involved in creating the coronavirus vaccine at the University of Oxford’s Jenner Institute have said that their vaccine candidate could be ready by September. For this to happen, clinical trials must be completed by the end of the summer.
In order to ensure that the vaccine is available by September, AstraZeneca, the biopharmaceutical company which is collaborating with scientists at Oxford University, has already started to manufacture the vaccine.
The University of Oxford, where research and development of the vaccine is taking place.
Should the vaccine be proven to be effective and safe, initial distribution of around 30 million doses can be made by September in the UK. The UK-based company has also agreed to make 400 million doses of the vaccine before the end of 2020.
There is currently no certainty that a vaccine will be ready in as little as 4 months, especially since there has yet to be any conclusive data from clinical trials. The Oxford University group itself has stated that this timeline is “highly ambitious and subject to change”. In order for the vaccine to be ready by autumn, the clinical trials must prove that the vaccine works as intended, and is safe for everyone to receive.