With the effective treatment of wounds from sores to lacerations, Pathelen Health Care is the company behind the Pathelen Hybrid medical device, a treatment that focuses on encouraging the healing process. It does this by ensuring that any biofilms that prevent the formation of new tissue are removed, reducing the risk of infection and increasing the effectiveness of the body’s ability to recover from the wound. All of this, as well as its client service abilities, has earned it the title of ‘2022’s Most Innovative Biotech Company’ in Switzerland & 2022’s Awards for Excellence in Innovation.
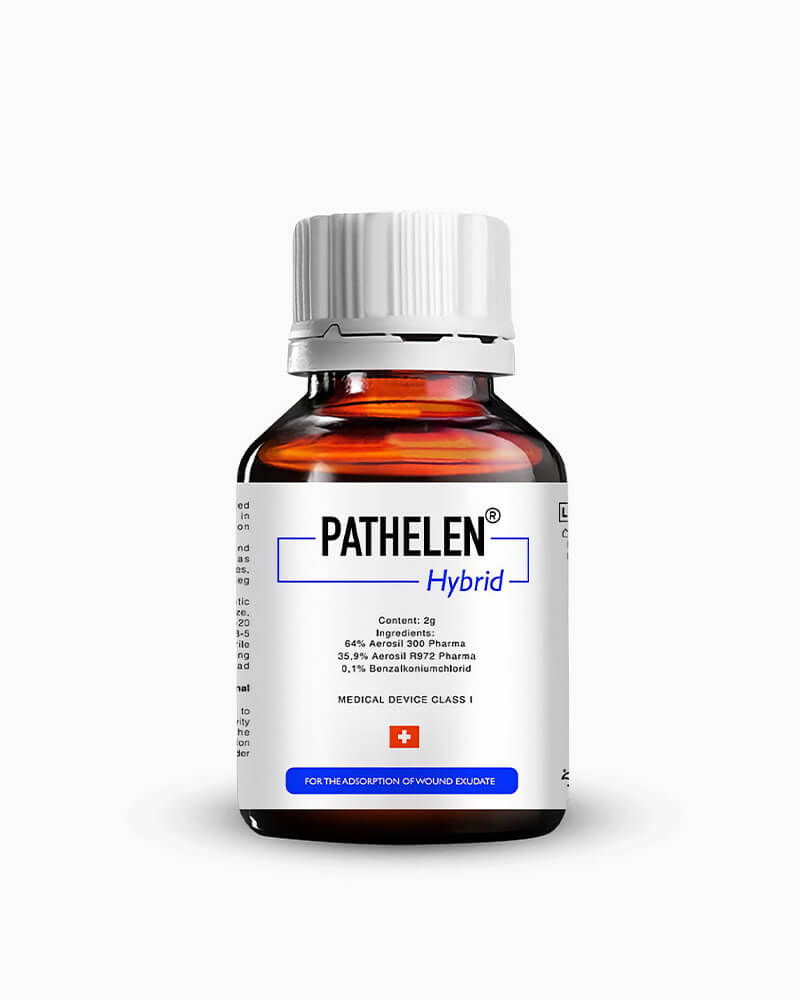
With a core flagship product around which everything else has been created, Pathelen Health Care’s focus has been on its Pathelen Hybrid since its inception. Nominally, Pathelen Hybrid is a medical device that has been registered with SwissMedic and the USA FDA as an exemplary device for use in non-healing chronic wounds. Built to stimulate the healing time of such wounds as diabetic foot ulcers, ulcus cruris, and bed sores, the Pathelen Hybrid has its uses in ensuring that potentially damaging wounds are taken care of in a way that avoids the condition of the body worsening. In this way, use of the device has prevented amputation by ensuring the wounds are taken care of in a timely manner.
Critically, as a superabsorber, this product’s main draw is its ability to deal with any discharged liquid that a wound creates, making sure that it doesn’t linger around the sore and cause increased danger of infection or sepsis. Hygienic, utilitarian, and safe to use, it ensures that every medical professional who comes into possession of the device knows how to use it, but has also worked hard to ensure that the device is not overly complicated in any way, shape or form. In healthcare – especially when dealing with open wounds – time is often of the essence, especially when amputation is at stake; thus, it has strived to cut the fat from the process.
Specifically, its main target is the removal of biofilms that prevent the formation of granulation tissue. First and foremost, the prevention of granulation tissue is what prevents a wound from closing and can mean additional danger for a patient in terms of the wound getting worse, making it a longer and more painful healing process. Thus, the superabsorbent properties of Pathelen Hybrid take the uncertainty out of it, ensuring that biofilms are kept to a minimum after its application once the wound is clean of debris.
Granulation tissue is normally formed after 3-5 days, which leads to a successful complete closure between 3 and 8 weeks depending on the size and severity of the laceration or sore. Moreover, with a success far above the norm for medical devices of its kind, it has gone above and beyond the expectations of the medical community for it at every turn, with no side effects observed and no added complications thanks to the use of the Pathelen hybrid device. Instead, patients it has been used on have reported back with successful wound closures within the forecasted time and a good experience with it that has allowed it to become a staple of the hospitals and medical facilities it has provided its medical devices to.
In this manner, it has been able to achieve its goals through its own innovations, developments, and tenacity, something that it will be continuing to do long into the future. This dedication to constant improvement has vastly further endeared it to the medical industry and the international medical community, as the smallest change in variable could be the difference between a patient keeping a limb and losing it.
If the Pathelen Hybrid is applied within a timely manner, its usefulness in saving life and limb truly cannot be overstated. Going forward, Pathelen looks forward to continuing to improve and redevelop the Hybrid as it grows as a company, ensuring that its clients and their patients are the ones who benefit from the ever-growing and changing developments involved in the wider world of medical science and its specific fields. A big part of this will be working to be able to address the treatment of chronic wounds with treatments that do not contain pharmaceutical products.
By addressing such things with medical devices instead of ingestible or bloodstream delivered products, a clinic or hospital can increase the amount of people their various investments can treat safely, as with a medical device there is far less risk of allergic reaction or adverse side effects. This non-pharmaceutical delivery method of treatments has, therefore, made itself a more and more popular staple of the modern medical industry, one that will only be seeing more and more investment as it moves forward into the future.
Moreover, its patented manufacturing process can achieve such things at a higher success rate and lower cost than most pharmaceutical products, making for a deeply trusted product that has thrust Pathelen into pre-eminence. With this notoriety has come further trust, respect, and custom from clinics, hospitals, health centres, and clinicians, something that it sees only increasing as time goes on, especially given its propensity for working to keep Pathelen Hybrid at the top of its game.
For business enquiries, contact Bijan Sabet from Pathelen Health Care via their website – pathelen-hybrid.com